· Hydrogen peroxide is a highly unstable chemical compound Two molecules of hydrogen combine with two molecules of oxygen to form hydrogen peroxide Hence, itsIt is an adduct of sodium carbonate ("soda ash" or "washing soda") and hydrogen peroxide (that is, a perhydrate) whose formula is more properly written as 2 Na 2CO 3 · 3 · Lewis structure for sodium peroxide Ask for details ;
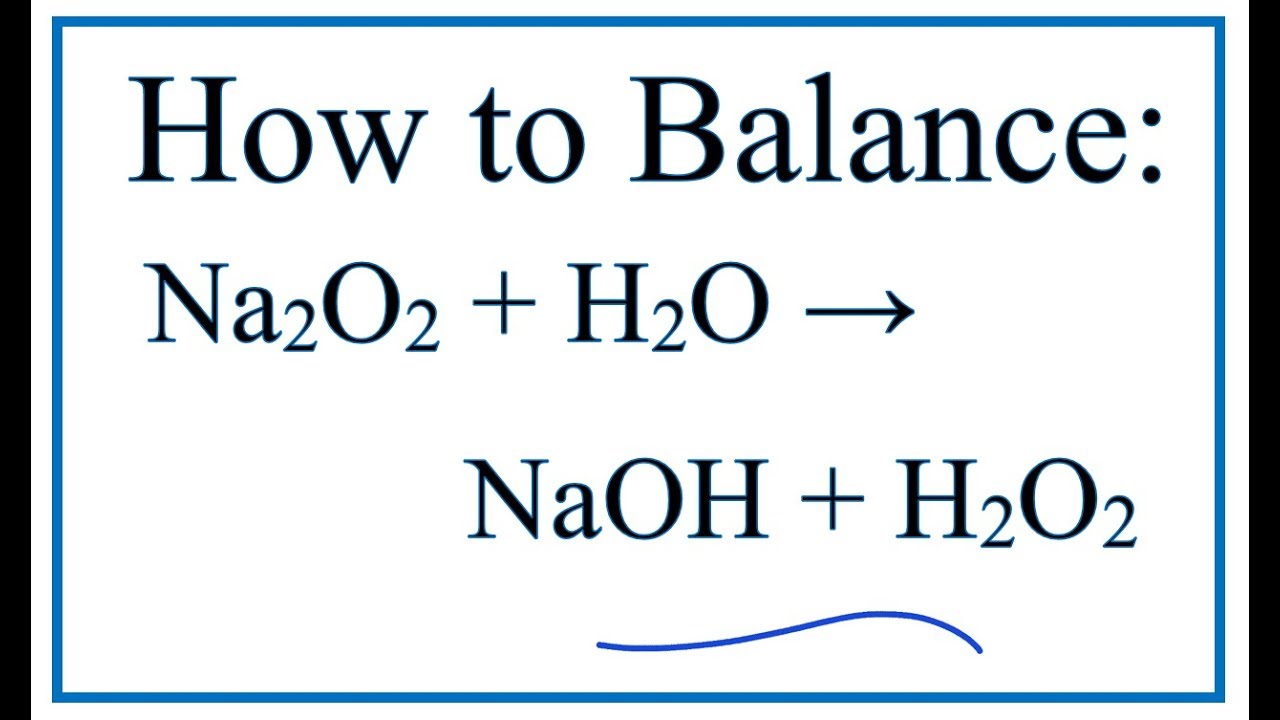
How To Balance Na2o2 H2o Naoh H2o2 Sodium Peroxide Cold Water Youtube
How to prepare sodium peroxide
How to prepare sodium peroxide-Buy Sodium Peroxide 25 Grams $ 125 Grams $177 Lab & Reagent Grade (≥930%) For Sale Online Formula Na2O2 YellowWhite To Yellow Granular SolidChemistry Q&A Library Oxidation of the chromium 3 ion (Cr* aq) by sodium peroxide (Na2) in alkaline solution produces sodium chromate (Na2CrO4) and hydroxide



Sodium Peroxide Market Size 21 Share Global Industry Growth Ktvn Channel 2 Reno Tahoe Sparks News Weather Video
Follow Report by Intelligentbrain9 Log in to add a commentSodium Peroxide Na2O2 Molar Mass, Molecular Weight • Na2O2 H2SO4 = Na2SO4 H2O2Sodium peroxide granular for analysis ACS,ISO CAS , pH 128 (100 g/l, H₂O, °C) Find MSDS or SDS, a COA, data sheets and more information
Sodium Carbonate Peroxide is the inorganic salt that conforms to the formula 2Na 2 CO 3 • 3H 2 O 2Structure of sodium peroxide Formula and structure The chemical formula of sodium hydroxide is NaOH, and its molar mass is 4001 g/mol TheSodium Peroxide is a highly insoluble thermally stable Sodium source suitable for glass, optic and ceramic applications Oxide compounds are not conductive to
The chemical formula for sodium peroxide given in the problem is a molecular formula, which tells us the number of atoms of each element per molecule As anSODIUM CARBONATE PEROXIDE can be found in 232 products Print Share on Evidence Health issue Level of Concern Source Component HYDROGEN PEROXIDE ATSDR states · Sodium oxide can be prepared via several routes The most common method involves burning sodium in air The reaction produces both sodium oxide and sodium


Sodium Carbonate Hydrogen Peroxide 2 1 1 Ch2na2o5 Chemspider


Materials Free Full Text Effect Of Sodium Hexametaphosphate And Trisodium Phosphate On Dispersion Of Polycarboxylate Superplasticizer Html
9 rows · Sodium peroxide appears as a yellowwhite to yellow granular solid Mixtures withSodium peroxide (Na2O2) hydrolyzes to give sodium hydroxide (NaOH) and hydrogen peroxide(H2O2) when it is treated with water according to the reaction Na2O2S odium peroxide is the inorganic compound with the formula Na 2 O 2 This yellowish solid is the product of sodium ignited in excess oxygen It is a strong



Sodiumoxide Sodium Sodium Hydroxide



Sodium Peroxide An Overview Sciencedirect Topics
7 rows · Molecular Formula NaO 2Synonyms NaO2 Molecular Weight 549 Component Compounds CIDSynonym Hydrogen peroxide sodium carbonate adduct Linear Formula Na 2 CO 3 ·15H 2 O 2 Molecular Weight CAS NumberSODIUM PEROXIDE reacts violently with reducing agents, combustible materials and light metals Reacts exothermically and rapidly or even explosively with water to form


Solved Toothpastes Containing Sodium Hydrogen Carbonate Sodium Bicarbonate And Hydrogen Peroxide Are Widely Used Write Lewis Structures For The Course Hero
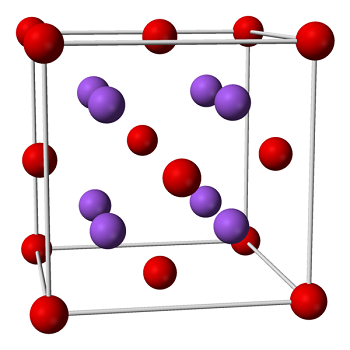


Sodium Oxide 1313 59 3
The oldest and previously most widely used bleaching agent is sodium perborate which hydrolyzes during use and forms hydrogen peroxide and borate Peroxide is completelyObjectives The mechanism of tooth bleaching using peroxide oxidizers is not fully understood It is unknown whether peroxide radicals make teeth whiter by · Sodium Oxide is produced with the reaction of sodium hydroxide and metallic sodium It can also be formed from sodium peroxide or sodium nitrate However
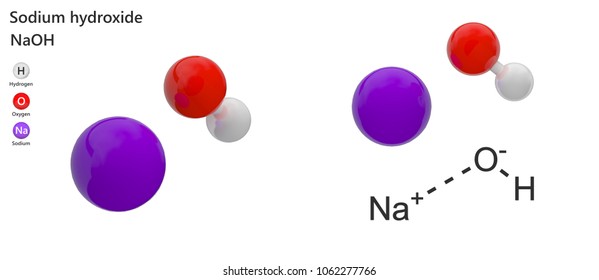


Sodium Hydroxide High Res Stock Images Shutterstock



Sodium Peroxide Cas 1313 60 6 Chemical Physical Properties By Chemeo
The table shows element percentages for NaO2 (sodium superoxide) A strong oxidizer An oxide is any chemical compound that contains one or more oxygen atoms SODIUMSODIUM PEROXIDE Na2O2 Properties of Sodium peroxide Na2O2 White (sometimes yellow because of the impurities NaO2) When heated in air turn yellow and decomposesSodium peroxide formula, also known as Disodium dioxide formula or Solozone formula a granular solid which is yellowwhite to yellow in colour Visit CoolGyan



Sodium Peroxide An Overview Sciencedirect Topics
.jpg)


Surfactants
/12/18 · sodium peroxide structure August 2, August 2, hungarian food products on sodium peroxide structure The compound has a melting point of ° C where it51 Sodium carbonate peroxyhydrate is the chemical name for an addition product produced by drying hydrogen 52 peroxide in the presence of sodium carbonate (CAS NoSodium peroxide definition, a yellowishwhite, hygroscopic, watersoluble powder, Na2O2, used chiefly as a bleaching agent and as an oxidizing agent See more



What Is Sodium Hydroxide Formula Reactions Video Lesson Transcript Study Com



How To Write The Formula For Naoh Sodium Hydroxide Youtube
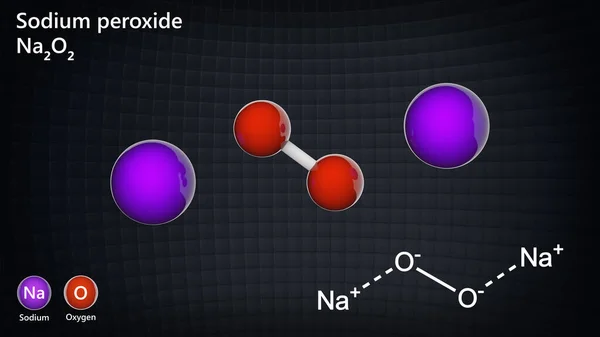
The molecular or chemical formula of Sodium peroxide is Na 2 O 2 Disodium dioxide is aMSLRPWGRFCKNIZUHFFFAOYSAJ Sodium carbonate peroxide Similar structures search, synonyms, formulas, resource links, and other chemical informationSodium peroxide is the inorganic compound with the formula Na₂O₂ This yellowish solid is the product of sodium ignited in excess oxygen It is a strong base This metal


Structural Chemical Formula And Molecular Structure Of Hydrogen Peroxide H2o2 Chemical Structure Model Ball And Stick 3d Illustration Larastock


Sodium Peroxide Wikipedia
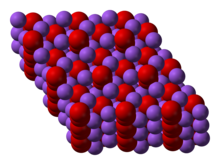
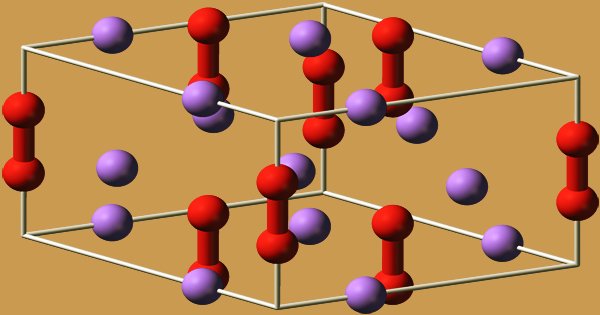
Solid state structure Geometry of sodium Prototypical structure Element analysis The table shows element percentages for Na 2 O 2 (sodium peroxide)Average mass Da;Monoisotopic mass Da;
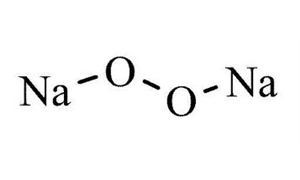


1313 60 6 Cas Sodium Peroxide Granular Peroxides Article No


Chemidplus 1313 60 6 Pfuvrdfdkpngav Uhfffaoysa N Sodium Peroxide Similar Structures Search Synonyms Formulas Resource Links And Other Chemical Information
Sodium peroxide, hydrogen peroxide, sodium perborate, and sodium persulfate are present in many labs Organic peroxides contain the bivalentOOWhile peroxide of hydrogen contains but three or four per cent of available bleaching oxygen, peroxide of sodium contains about twenty per cent, and as aChemistry Q&A Library Sodium chromate can be prepared by oxidizing a chromium(III) salt with sodium peroxide in alkaline solution The chromium(III) ions are



Write The Formula Using Criss Cross Method For Sodium Hydroxide Brainly In



Welcome To Chem Zipper Com What Is The Sodium Per Borate Give The Structure And Its Uses
Sodium peroxide is a chemical compound with the formula Na2O2 that has two ionic bonds between the two sodium atoms and the O2 molecule It exists in variousThe negatively charged peroxide ion (O 2 2) is present in inorganic compounds that may be regarded as salts of the very weak acid hydrogen peroxide;Sodium peroxide Molecular Formula Na 2 O 2;


Sodium Peroxide Properties Uses Assignment Point
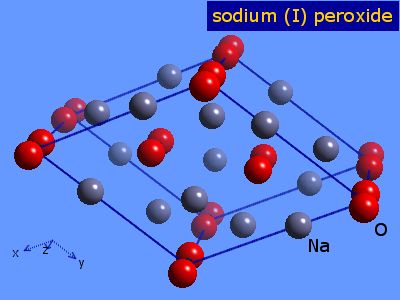


Webelements Periodic Table Sodium Sodium Peroxide
Sodium peroxide Na2O2 structure 🎓Molecular Formula Na2O2 Average mass Da Density Boiling Point Flash Point Molar Refractivity Polarizability SurfaceSodium percarbonate The name "sodium percarbonate" (SPC) does not reflect the structure of this oxidizing agent, which is in fact a carbonate perhydrate 2 Na 2Characteristics and gel properties of gelatin from goat skin as affected by pretreatments using sodium sulfate and hydrogen peroxide Sulaiman MadAli et al Journal



Epb1 Stabilized Hydrogen Peroxide Solutions Google Patents



Sodium Peroxide An Overview Sciencedirect Topics
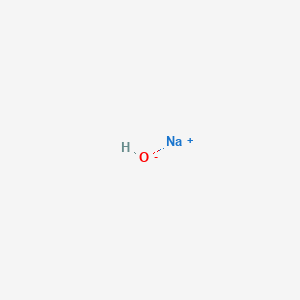


Sodium Hydroxide Naoh Pubchem



Sodium Peroxide Wikiwand



Chemical Reaction Precipitation Reactions Britannica



Sodium Peroxide 96 Acros Organics 25g Sodium Peroxide 96 Acros Organics Fisher Scientific



Scheme 8 Mechanism Of Interaction Luminol With Peroxide And Sodium Download Scientific Diagram



Unveiling The Charge Migration Mechanism In Na 2 O 2 Implications For Sodium Air Batteries Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cph



Peroxide



Relationship Between Structure And Catalyst Effect In The Degradation Kinetics Of Reactive Dyes With Hydrogen Peroxide Uv Light Versus Sodium Hydroxide Sciencedirect


60 Sodium Hydroxide Vectors Royalty Free Vector Sodium Hydroxide Images Depositphotos
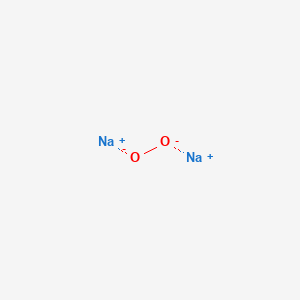


Sodium Peroxide Na2o2 Pubchem



Sodium Peroxide


Sodium Hypochlorite Podcast Chemistry World



Sodium Peroxide Market Size 21 Share Global Industry Growth Ktvn Channel 2 Reno Tahoe Sparks News Weather Video



How To Balance Na2o2 H2o Naoh H2o2 Sodium Peroxide Cold Water Youtube


Sodium Perborate


Sodium Peroxide Na2o2 Chemspider


Webelements Periodic Table Sodium Sodium Iodide


Dichloroethane


Hydrogen Peroxide Structure Uses And Properties Of Hydrogen Peroxide


Sodium Peroxide 1313 60 6 France Sodium Peroxide 1313 60 6 Manufacturers France Sodium Peroxide 1313 60 6 Suppliers
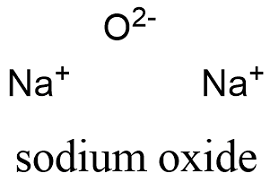


Sodium Oxide Formula


Calcium Peroxide Wikipedia
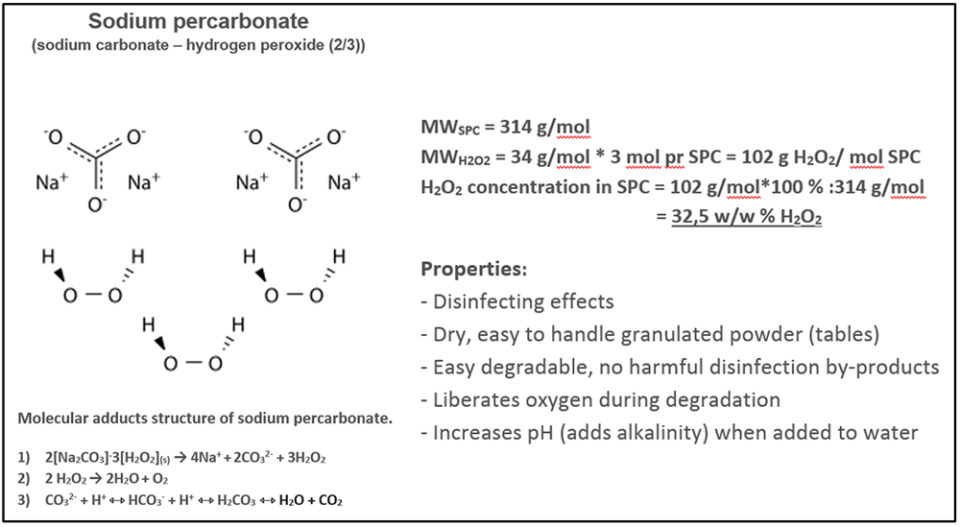


The Pros And Cons Of Sodium Percarbonate Global Aquaculture Advocate



Structure Of Oxidizing Agents A Sodium Hypochlorite B Download Scientific Diagram


Why Can Na2o2 Not Be Written As Nao Quora


Organic Syntheses Procedure
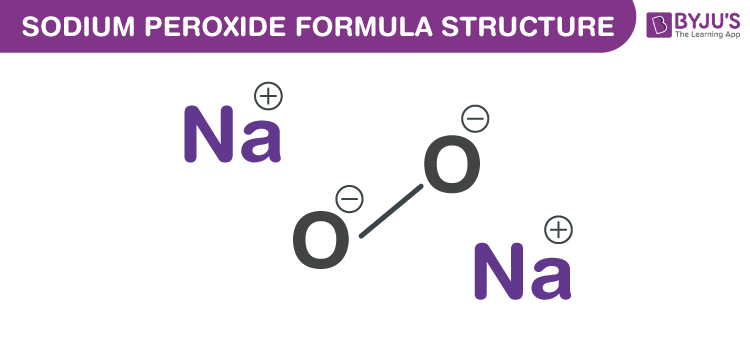


Sodium Peroxide Formula Chemical Formula Structure And Properties
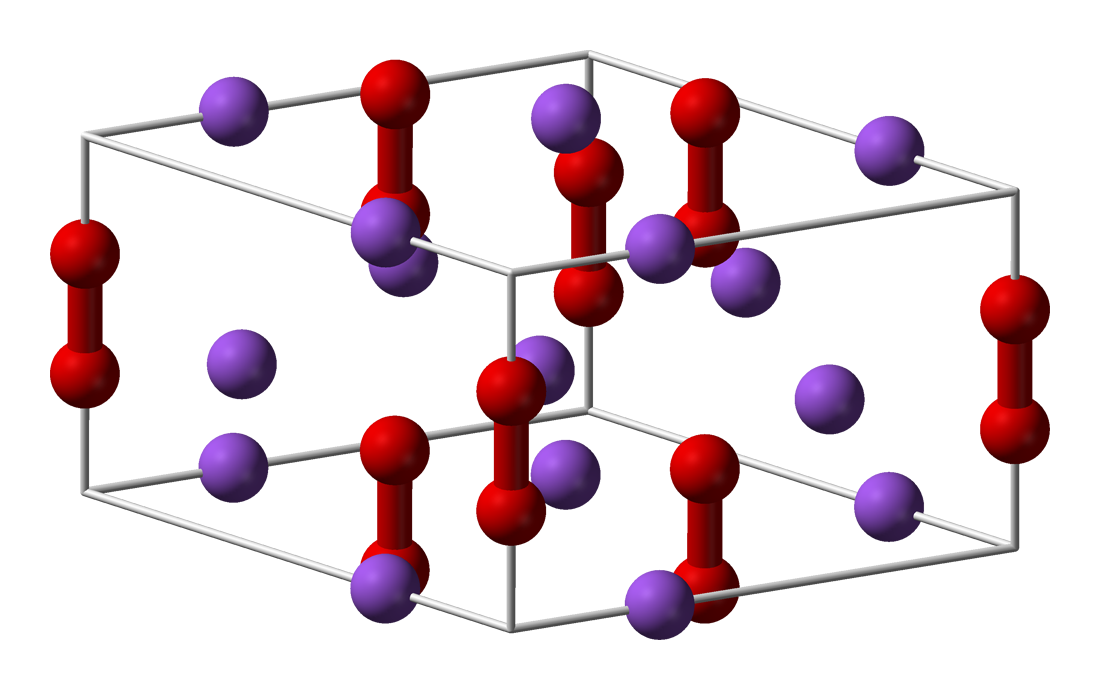


Sodium Peroxide Wikipedia


Sodium Peroxide



Figure 2 From Intrinsic Conductivity In Sodium Air Battery Discharge Phases Sodium Superoxide Vs Sodium Peroxide Semantic Scholar



Sodium Hydroxide Wikipedia
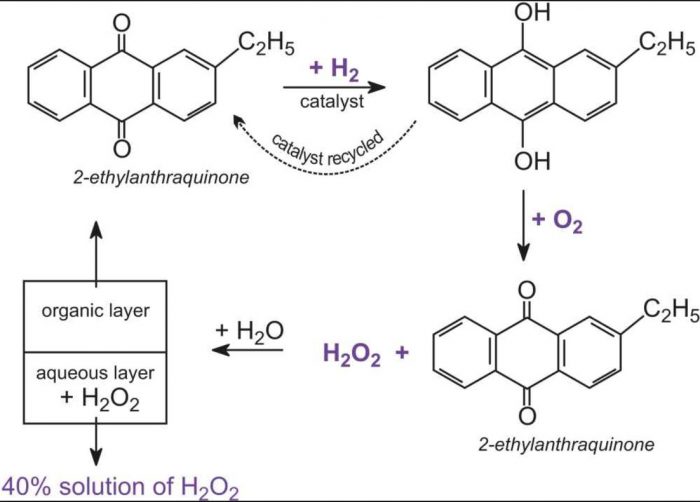


Hydrogen Peroxide Chemistry Class 11 Hydrogen
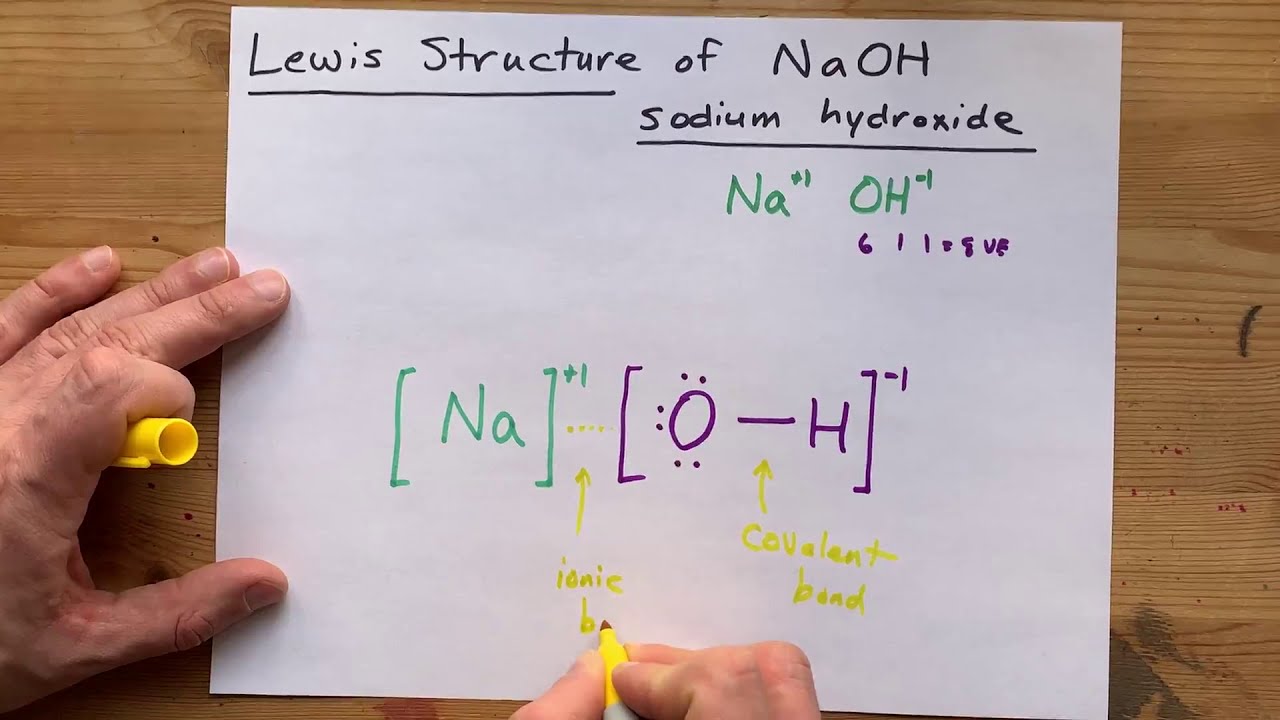


Lewis Structure Of Naoh Sodium Hydroxide Youtube



Sodium Peroxide Honeywell Research Chemicals



What Happens When Sodium Peroxide Dissolves In Water
/DCFABEB1E647E059802585F900812BB3/$file/FS03634_structure.png)


Sodium Peroxide 1313 60 6 Carbosynth Product



What Is Sodium Hydroxide Formula Reactions Video Lesson Transcript Study Com



Nah2po4 Sodium Dihydrogen Phosphate Structure Properties And Uses
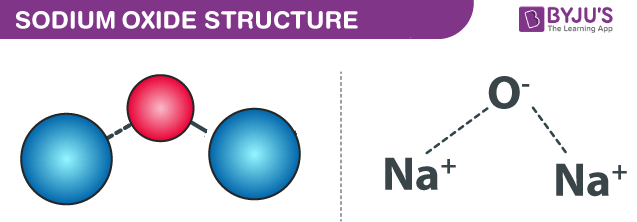


Sodium Oxide Na2o Structure Properties Uses Faqs



Kra Process For Preparing Divinylarene Dioxides Google Patents



How To Find The Oxidation Number For O In Na2o2 Sodium Peroxide Youtube
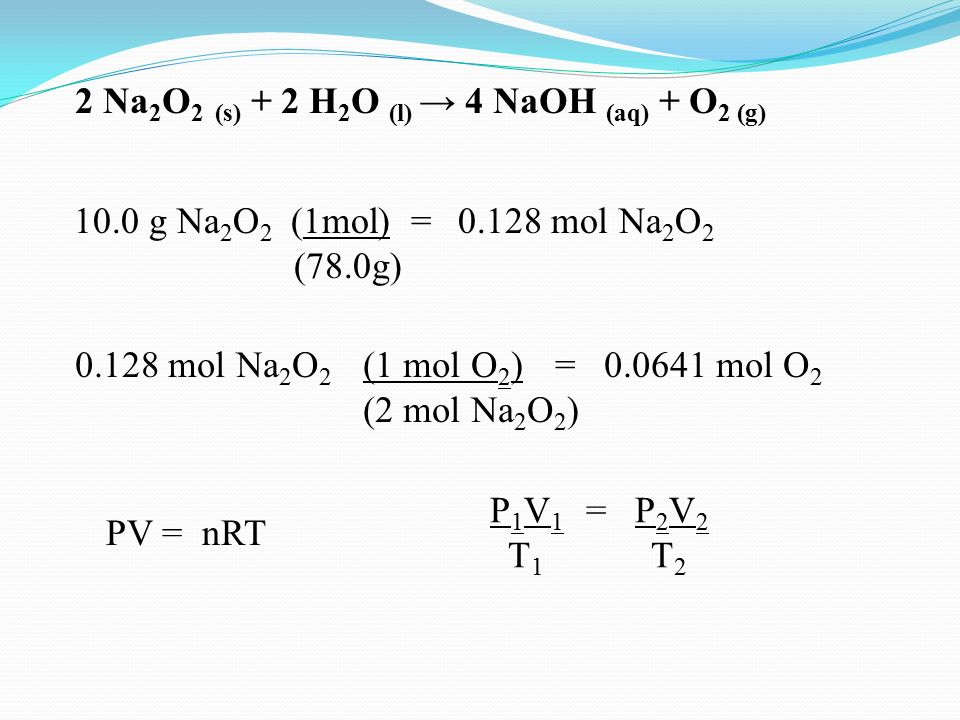


Practice Problem If 10 0 G If Sodium Peroxide Na2o2 Reacts With Water To Produce Sodium Hydroxide And Oxygen How Many Liters Of Oxygen Will Be Produced Ppt Video Online Download



Sodium Peroxide Market Outlook Trend And Opportunity Analysis Competitive Insights Actionable Segmentation Forecast 26 The Courier
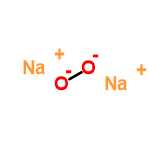


Sodium Peroxide Na2o2 Structure Studyhippo Com


What Is The Name Of Na2o2 Quora



Toothpastes Containing Sodium Hydrogen Car Clutch Prep


What Is The Oxidation Number Of Oxygen In Na2o2 Quora


Peroxide Wikipedia



Question Video Determining The Empirical Formula Of Sodium Peroxide Na O Nagwa


18 9 Occurrence Preparation And Compounds Of Oxygen Chemistry



Sodium Peroxide Acs Reagent 93 0 1313 60 6 Sigma Aldrich


Lewis Structure Of Sodium Peroxide Cad Vigyan


Sodium Alginate


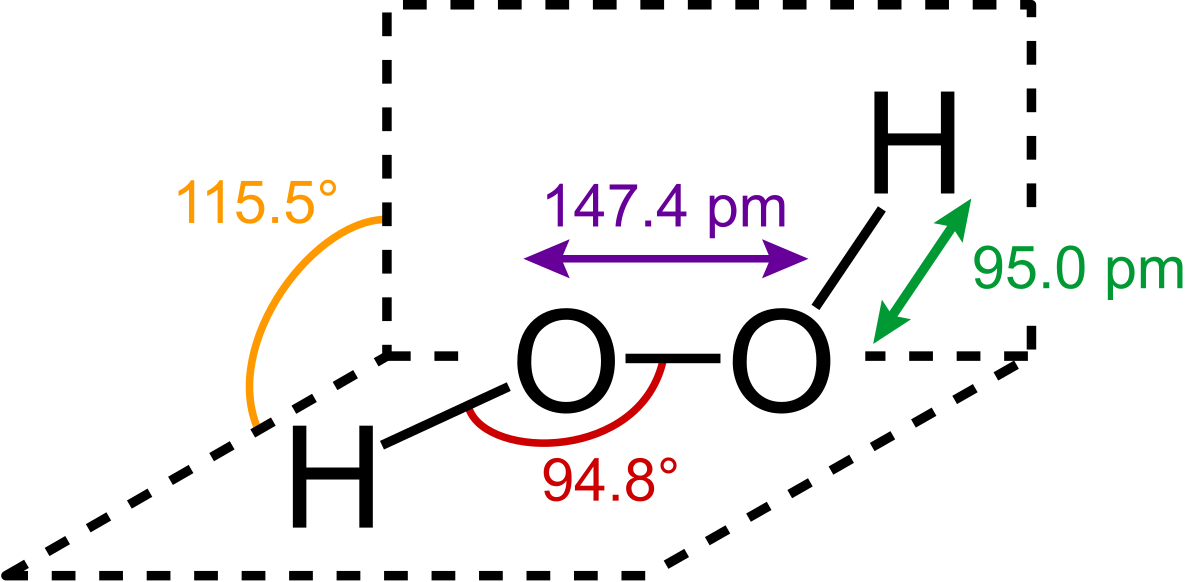
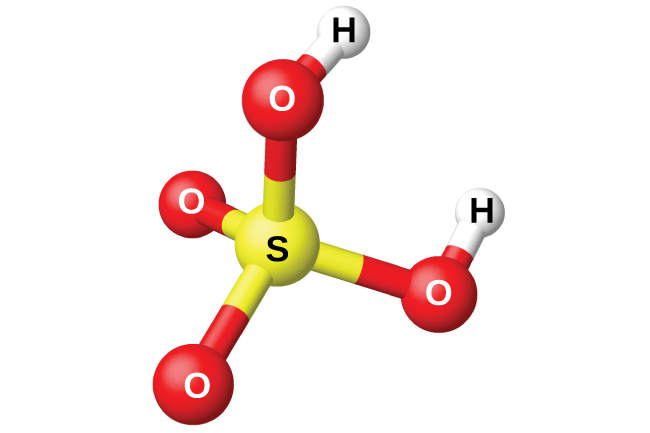
Solved 3 This Question Is About Compounds Of Sodium A Chegg Com


Sodium Peroxide Na2o2 Pubchem
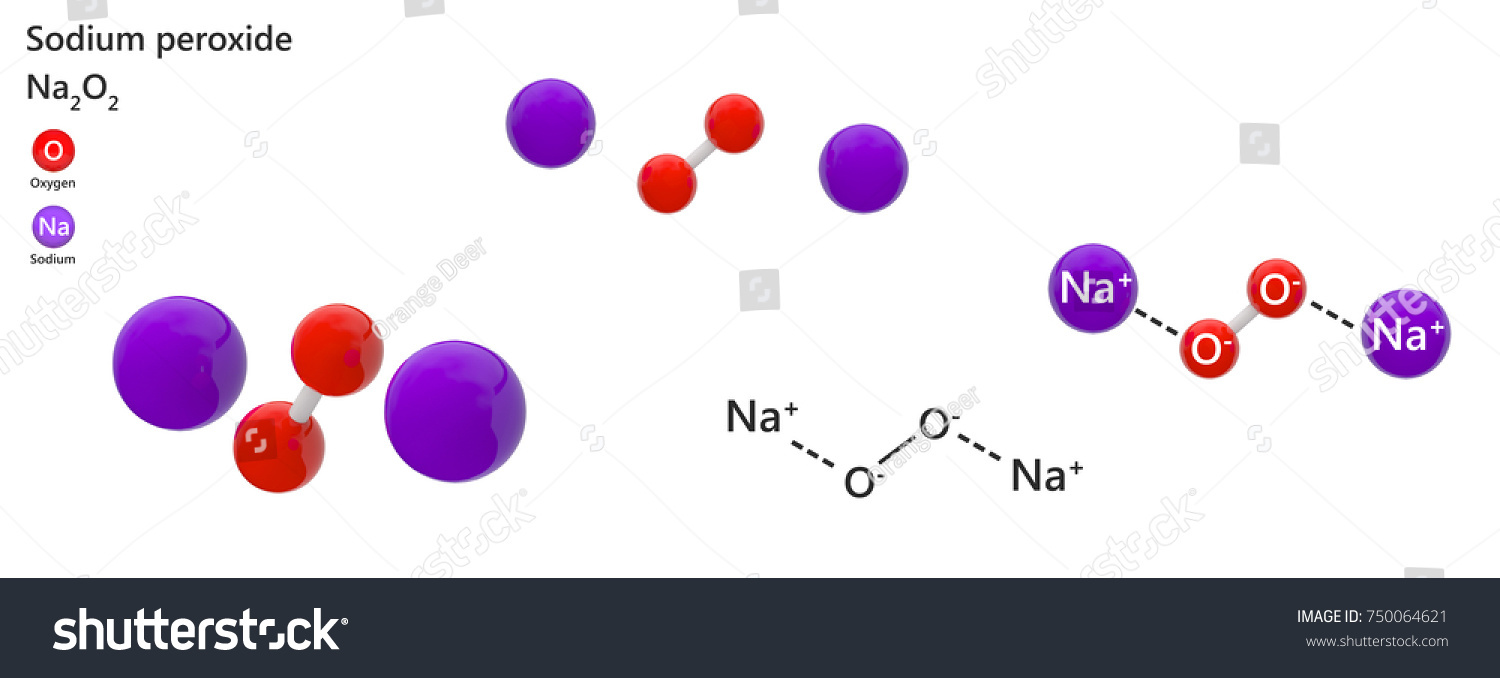


Sodium Peroxide Inorganic Compound Formula Na2o2 Stock Illustration


Na2o2 图片 库存照片和矢量图 Shutterstock



Sodium Peroxide Youtube



What Is Caustic Soda



Sodium Peroxide Youtube



Sodium Peroxide 95 Cas 1313 60 6 Glentham Life Sciences
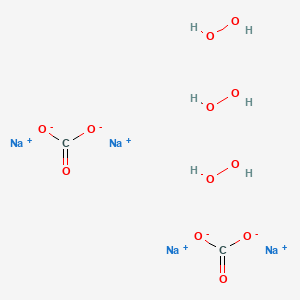


Sodium Percarbonate C2h6na4o12 Pubchem
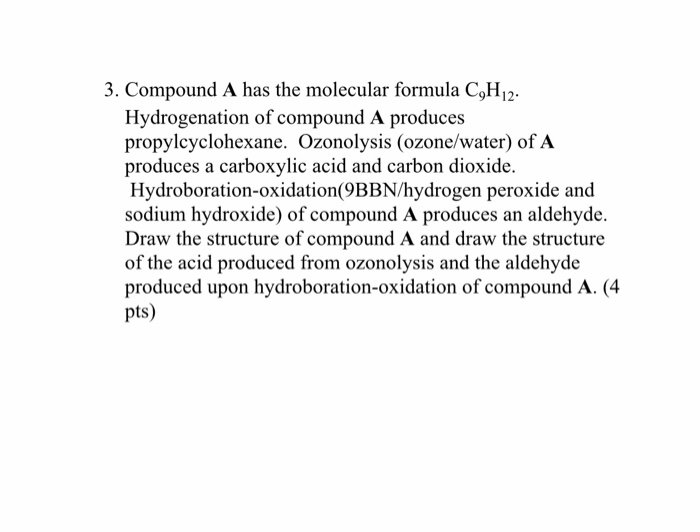


Solved 3 Compound A Has The Molecular Formula C H 91j2 Chegg Com
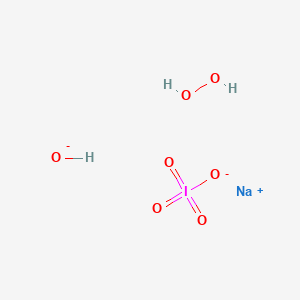


Periodate Sodium Hydroxide Hydrogen Peroxide H3inao7 Pubchem
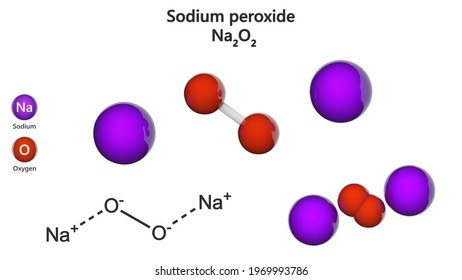


Disodium High Res Stock Images Shutterstock



Sodium Peroxide An Overview Sciencedirect Topics
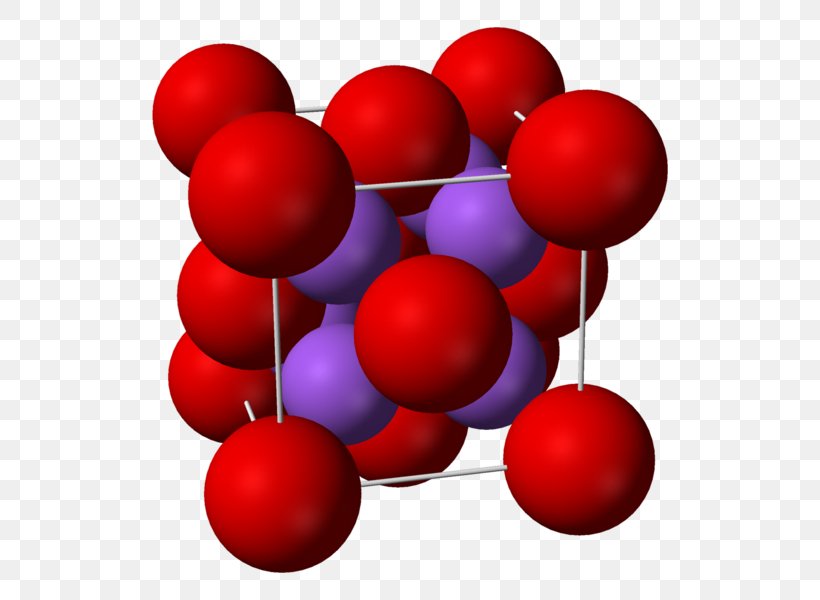


Sodium Oxide Calcium Oxide Sodium Peroxide Png 570x600px Sodium Oxide Calcium Oxide Chemical Bond Chemistry Crystal



Hydrogen Peroxide 30 Stabilized With Sodium Stannate Certified Fisher Chemical Fisher Scientific


How Does Bleach Work Chemically
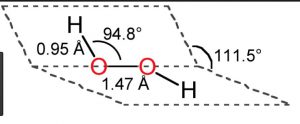


Hydrogen Peroxide Chemistry Class 11 Hydrogen



Hydrogen Peroxide Preparation Properties Structure And Uses Definition Examples Diagrams



Nickel Hydroxides And Related Materials A Review Of Their Structures Synthesis And Properties Proceedings Of The Royal Society A Mathematical Physical And Engineering Sciences



0 件のコメント:
コメントを投稿